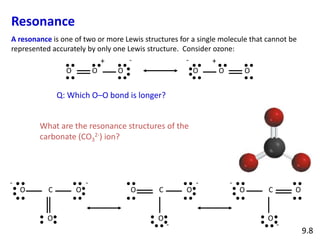
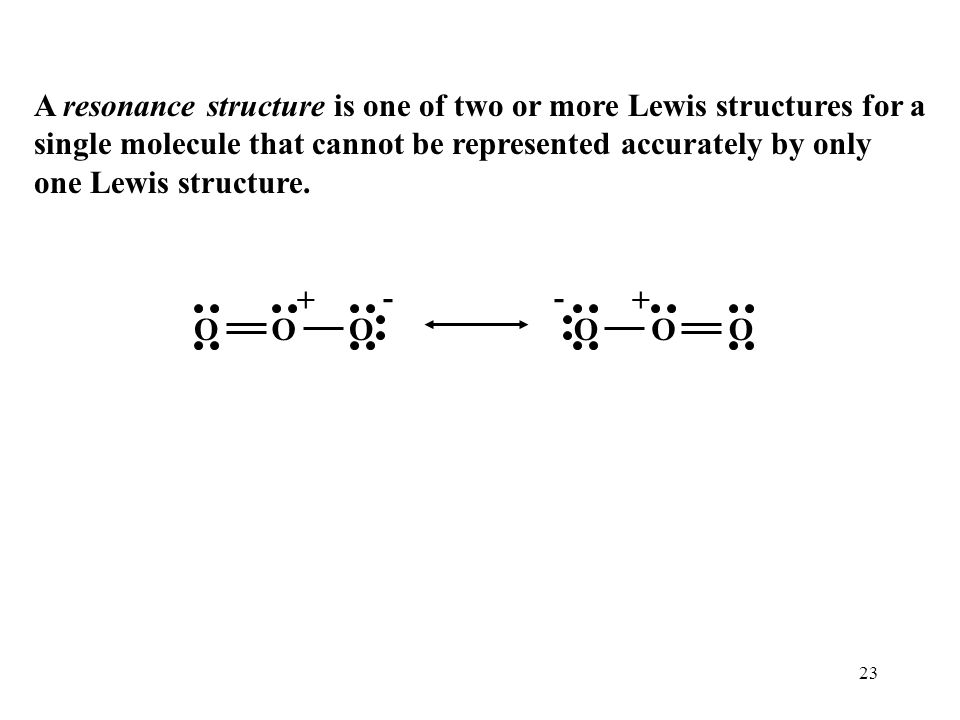
Explain why CO_(3)^(2-) ion cannot be represented by a single Lewis structure. How can it be best represented?

Explain why CO_{3}^{2-} ion cannot be represented by a single Lewis structure. How can it be best represented?
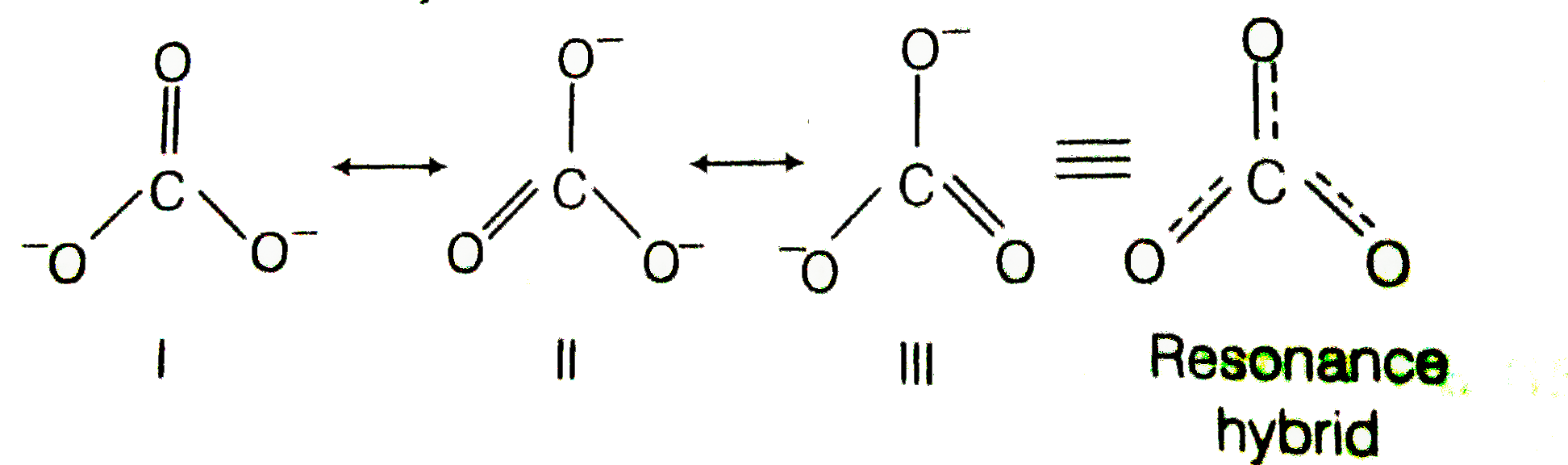
Explain why CO_(3)^(2-) ion cannot be represented by a single Lewis structure. How can it be best represented?

Simplified S 0 singlet PES at the G3SX//M06-2X/aug-cc-pVTZ level of... | Download Scientific Diagram
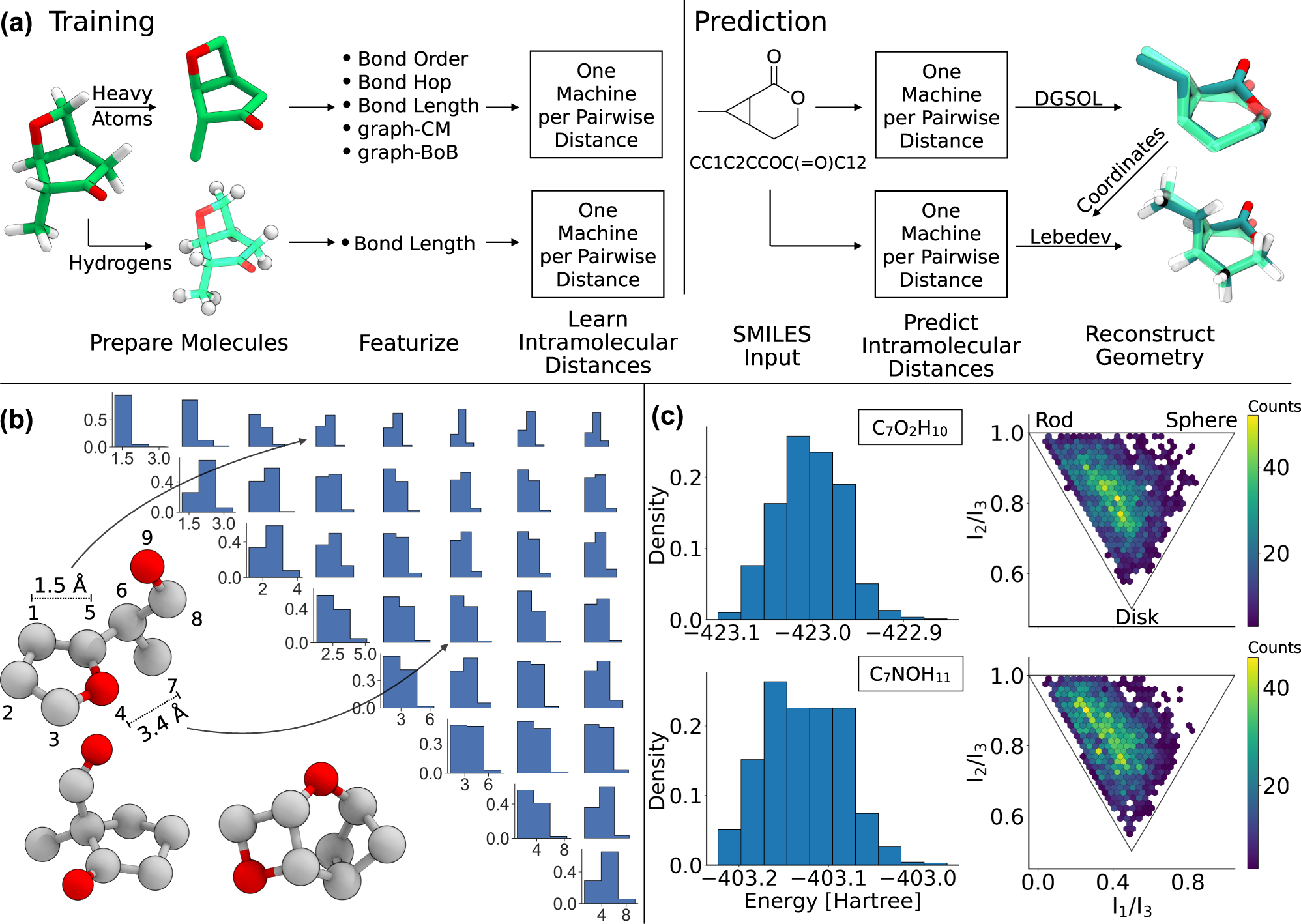
Machine learning based energy-free structure predictions of molecules, transition states, and solids | Nature Communications

Explain why CO_(3^(2-) ion cannot be represented by a single Lewis structure. How can it be best represented?

Question 44 Explain why CO 32- ion cannot be represented by a single Lewis structure. How can it be best represented?



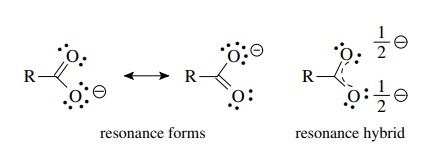
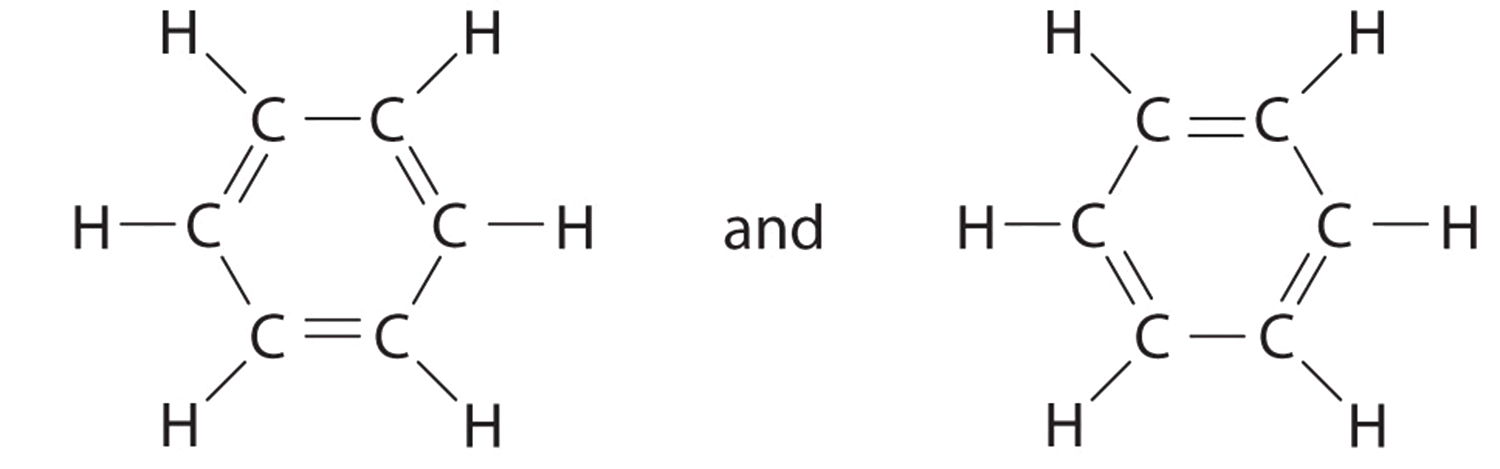









/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)